For someone living with rheumatoid arthritis (RA), mornings can feel like a battle. Joints stiff, swollen, and burning - even simple tasks like opening a jar or stepping out of bed become exhausting. For decades, RA was seen as an inevitable march toward joint destruction. But today, that story is changing. Thanks to biologic DMARDs, remission isn’t just a hope - it’s a realistic outcome for many.
What Are Biologic DMARDs and How Do They Work?
Biologic DMARDs, or biologic disease-modifying antirheumatic drugs, are targeted therapies designed to block specific parts of the immune system that drive inflammation in RA. Unlike older drugs like methotrexate that suppress the whole immune system, biologics zero in on the culprits - proteins like TNF-alpha, IL-6, or molecules on T-cells and B-cells that trigger joint damage. The first biologic for RA, etanercept (Enbrel), hit the market in 1998. Since then, the toolbox has grown. Today, there are TNF inhibitors like adalimumab (Humira), infliximab (Remicade), and golimumab (Simponi). Then there are non-TNF options: abatacept (Orencia) that blocks T-cell activation, rituximab (Rituxan) that depletes B-cells, and tocilizumab (Actemra) that shuts down IL-6 signaling. Even newer are JAK inhibitors like tofacitinib (Xeljanz) and upadacitinib (Rinvoq), which work inside cells to interrupt inflammation signals. These drugs don’t just ease pain. They stop the erosion of bone and cartilage. In clinical trials, 20-50% of patients on biologics achieve remission - compared to just 5-15% on methotrexate alone. That’s not a small difference. It’s the difference between living with constant pain and living without it.Who Gets Biologic DMARDs - And When?
Biologics aren’t the first line of defense. The American College of Rheumatology and other major guidelines still recommend methotrexate as the starting point for most RA patients. It’s cheap, well-studied, and works for many. But if after 3-6 months, symptoms haven’t improved - or joint damage is already visible on X-rays - doctors move to biologics. This isn’t about severity alone. It’s about response. If you’re still swollen, fatigued, and struggling after methotrexate, you’re a candidate. Around 30% of RA patients eventually need a biologic. That number rises to nearly half in those with high disease activity at diagnosis. Treatment often combines a biologic with methotrexate. Studies show this combo works better than either drug alone. But some patients can’t tolerate methotrexate due to side effects - liver issues, nausea, or lung sensitivity. In those cases, biologics can be used alone.How Effective Are Different Biologics?
Not all biologics are created equal. A 2022 review in Exploration Medicine found that adalimumab, etanercept, and golimumab were about 19% more effective than infliximab in real-world settings. But here’s the twist: non-TNF biologics like abatacept and tocilizumab often outperform TNF inhibitors in certain patients. Why? Because RA isn’t one disease. It’s many. Some patients have high levels of B-cells in their joints - those respond better to rituximab. Others have excess IL-6 - tocilizumab works wonders for them. And if your immune system is driven by T-cell overactivity, abatacept may be the best fit. Even more telling: biomarkers can predict response. One study showed that patients with low synovial B-cell signatures had only a 12% chance of responding to rituximab - but a 50% chance with tocilizumab. This isn’t guesswork anymore. Doctors are starting to use joint fluid or blood tests to guide choices. JAK inhibitors like upadacitinib have shown even higher remission rates in head-to-head trials against adalimumab. In the SELECT-COMPARE study, nearly 40% of patients on upadacitinib achieved remission at 6 months - compared to 25% on adalimumab.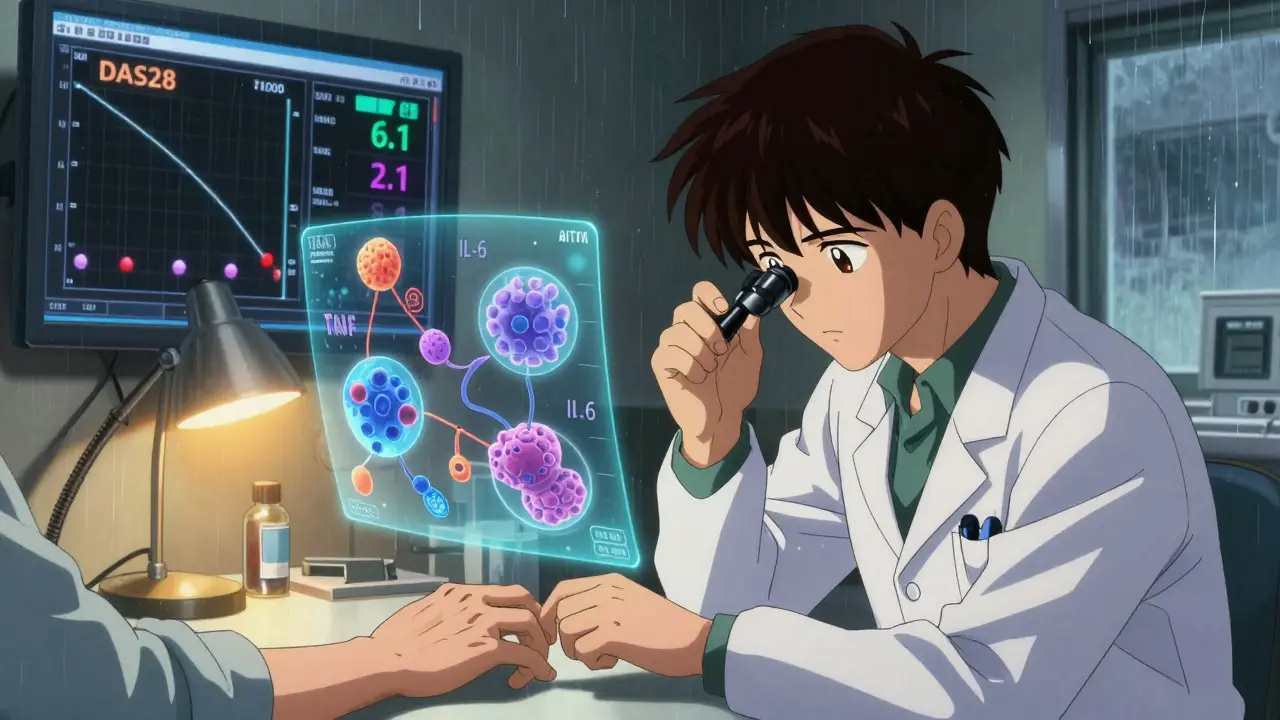
Side Effects and Risks
Biologics aren’t risk-free. Because they suppress parts of the immune system, infections become more common. Tuberculosis, pneumonia, and skin infections are the biggest concerns. That’s why everyone gets a TB test before starting. Some patients develop serious infections requiring hospitalization - about 1 in 20 over a year. Injection site reactions are common with subcutaneous drugs like adalimumab and etanercept. Redness, itching, or swelling at the injection spot affects up to 45% of users. But most cases are mild and fade with time. There’s also a small increase in risk for certain cancers, particularly lymphoma. The absolute risk remains low - about 0.1-0.2% per year - but it’s something to discuss with your doctor, especially if you have a history of cancer. And then there’s the cost. In the U.S., a year of biologic therapy can run $50,000-$70,000. That’s why many patients turn to biosimilars - copies of original biologics that cost 15-30% less. By 2023, biosimilars made up 35% of TNF inhibitor prescriptions in the U.S. Many patients report similar results with biosimilars, though some worry about switching between brands. Real-world data shows most do fine, but close monitoring is key.What Does Remission Really Look Like?
Remission doesn’t mean RA is gone. It means the disease is quiet - no swelling, no pain, no signs of active inflammation on blood tests or scans. Doctors use tools like DAS28 (Disease Activity Score) to measure it. A score below 2.6 means remission. Patients who reach remission often describe it as a rebirth. One woman, diagnosed with severe RA at 32, started tocilizumab in 2021. Within eight weeks, her swollen hands returned to normal. She went back to gardening. She stopped using canes. She told her doctor, “I feel like I got my life back.” But remission isn’t permanent for everyone. About 40% of patients experience secondary non-response - the drug stops working after 12-24 months. That’s when switching to another biologic or JAK inhibitor becomes necessary. Studies show the benefit of each new biologic drops with each try. The first one often works best.
How to Take Biologics - And Stick With Them
Most biologics are injected under the skin - weekly or every other week. A few, like infliximab, require IV infusions every 4-8 weeks at a clinic. Self-injection sounds scary, but most patients master it in 1-4 weeks. The Arthritis Foundation reports 75% of patients succeed after just two training sessions with a nurse. Sticking to the schedule is critical. Missing doses lowers effectiveness and increases the chance of your body developing antibodies against the drug - which can make it stop working entirely. Insurance approval can be a hurdle. It often takes 7-14 days to get authorization. Some patients wait weeks just to start. Manufacturer patient assistance programs can cover 40-100% of costs for those who qualify. Specialty pharmacies handle delivery, storage (many need refrigeration), and education. Digital tools like ArthritisPower and MyRApath help track symptoms, side effects, and medication logs. These aren’t just apps - they’re lifelines for doctors trying to fine-tune treatment.The Future of RA Treatment
The next five years will bring big changes. Longer-acting biologics are in development - including a twice-yearly version of tocilizumab now in Phase III trials. That could mean just two injections a year instead of 26. Biosimilars will keep growing. By 2027, they’re expected to make up 60% of the biologic RA market. That’s good news for patients and healthcare systems. The biggest breakthrough? Personalized medicine. Researchers are now using synovial tissue analysis and blood biomarkers to match patients with the right drug before they even start. Imagine knowing, before your first injection, whether adalimumab or rituximab will work best for you. That’s not science fiction - it’s already happening in research centers. And while cost remains a barrier - especially in developing countries - global access is slowly improving. In the U.S. and Western Europe, 25-30% of RA patients use biologics. In low-income nations, it’s under 10%. That gap won’t close overnight, but biosimilars and advocacy are moving the needle.Final Thoughts
Rheumatoid arthritis used to be a sentence. Now, it’s a condition you can manage - even silence. Biologic DMARDs turned the tide. They didn’t just reduce pain. They gave people back their lives. But they’re not magic. They require commitment, monitoring, and sometimes tough choices. The goal isn’t just to feel better today - it’s to stay well for decades. With the right drug, the right support, and the right mindset, remission isn’t a dream. It’s your next step.Can biologic DMARDs cure rheumatoid arthritis?
No, biologic DMARDs cannot cure rheumatoid arthritis. RA is a chronic autoimmune condition, and there is currently no known cure. However, biologics can induce disease remission - meaning inflammation and symptoms are suppressed to the point where daily life is no longer disrupted. Many patients maintain remission for years with continued treatment, but stopping the medication often leads to a flare-up.
How long does it take for biologic DMARDs to work?
TNF inhibitors like adalimumab and etanercept often start working within 2-4 weeks, with noticeable symptom relief by 6-8 weeks. Non-TNF biologics like abatacept and tocilizumab may take longer - sometimes up to 12 weeks - to show full effect. JAK inhibitors like upadacitinib can work faster, with improvements seen in as little as 2 weeks. Patience is key, but if no improvement is seen after 3 months, your doctor may consider switching therapies.
Are biosimilars as effective as the original biologics?
Yes, biosimilars are just as effective as their originator biologics. The FDA requires them to have no clinically meaningful differences in safety, purity, or potency. Real-world studies and patient reports confirm similar outcomes in symptom control and remission rates. Many patients switch successfully from Humira to its biosimilar, for example, with no loss of benefit. Insurance companies often push biosimilars because they’re 15-30% cheaper.
What happens if a biologic stops working?
If a biologic loses effectiveness - often after 12-24 months - it’s called secondary non-response. This can happen due to the body developing antibodies against the drug or changes in disease activity. The next step is usually switching to another biologic with a different mechanism of action. For example, if a TNF inhibitor fails, switching to a JAK inhibitor or IL-6 blocker often works. Sequential use of multiple biologics reduces effectiveness with each try, so choosing the right first option matters.
Do I need to take biologics forever?
Most patients need to take biologics long-term to maintain remission. Stopping treatment increases the risk of flare-ups by over 70% within a year. However, a small subset of patients - usually those who achieve deep, sustained remission and have low disease activity markers - may be able to taper off under close supervision. This is rare and must be done with expert guidance. Never stop a biologic without consulting your rheumatologist.

14 Comments
Jocelyn Lachapelle December 15 2025
I was diagnosed with RA at 28 and thought I'd never garden again. Biologics gave me back my hands. No more canes. No more mornings spent crying from pain. I'm 42 now and I grow tomatoes like it's my job.
Lisa Davies December 16 2025
This made me cry 😭 I started adalimumab last year and now I can hold my baby without wincing. Thank you for writing this. So many people don't get it.
Nupur Vimal December 17 2025
In India most people still use methotrexate because biologics cost more than a car. You think remission is possible but for us it's a fantasy. Doctors here don't even know what DAS28 is
Melissa Taylor December 18 2025
I switched from Humira to its biosimilar last year. Same results. Same side effects. Half the price. Why are we still paying full price when the science says it's identical?
Christina Bischof December 18 2025
I used to think biologics were magic. Turns out they're just really expensive tools. The real win is finding the right one. Took me three tries. My rheum doc finally got it right with abatacept. Life changed.
Michelle M December 20 2025
It's not about curing. It's about reclaiming. RA steals your autonomy. Biologics don't fix your joints. They give you back the choice to get out of bed. That's the real miracle.
Sai Nguyen December 20 2025
Americans spend too much on medicine. In my country we use traditional herbs. No injections. No side effects. You just need discipline.
RONALD Randolph December 22 2025
Biosimilars? Are you kidding? The FDA's approval process is a joke. You can't replicate a biologic. It's a living molecule. You're playing Russian roulette with your immune system.
Benjamin Glover December 23 2025
The real issue isn't the drug. It's the patient's adherence. Most fail because they miss doses. Or they think they're cured after six months. Medical ignorance is the true epidemic.
Raj Kumar December 23 2025
My doc said try rituximab because my joint fluid had high B-cell count. Worked like a charm. Took me 3 months to feel it but now I'm in remission. Biomarkers are the future for sure.
Mike Nordby December 24 2025
The data on JAK inhibitors is compelling. The SELECT-COMPARE trial showed a 40% remission rate at six months. That's statistically significant and clinically meaningful. We need more head-to-head studies.
John Samuel December 25 2025
Biologics didn't just change my life-they resurrected it. I went from wheelchair to marathon training. Not because I'm special. Because science finally caught up with my pain. The future is bright, but we must fight for access.
Cassie Henriques December 26 2025
I track everything in ArthritisPower. My DAS28 dropped from 5.8 to 1.9 in 4 months on upadacitinib. My rheum doc uses my logs to adjust dosing. Tech + meds = game changer.
Jake Sinatra December 26 2025
The biggest barrier isn't cost or efficacy. It's stigma. People think RA is just old age pain. They don't see the fatigue. The brain fog. The grief. We need better public education, not just better drugs.